|
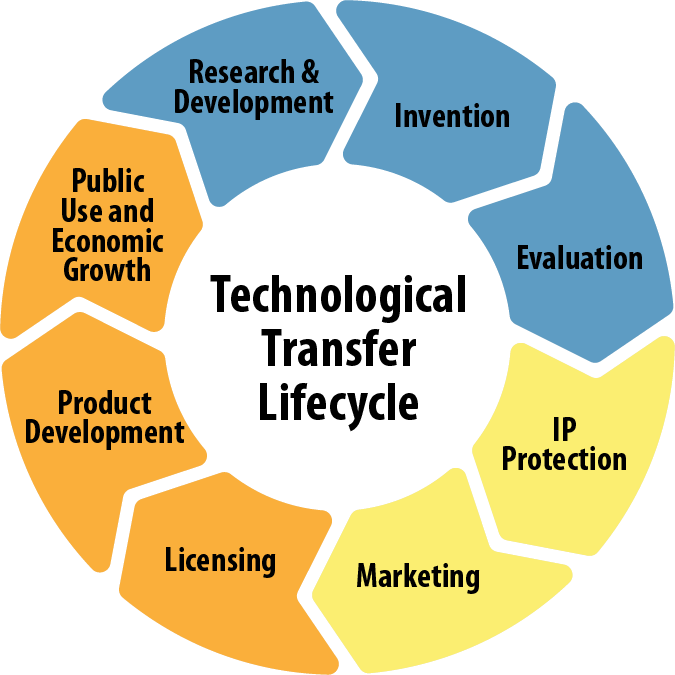
Il Technology Transfer Office (l’Ufficio per il Trasferimento Tecnologico - TTO), istituito nel 2017 sotto la Direzione Scientifica, svolge attività di supporto per la protezione, lo sfruttamento e il trasferimento delle scoperte dei ricercatori e del personale sanitario dell'IRCCS INRCA verso le imprese.
Il TTO incoraggia la protezione del know-how e della proprietà intellettuale attraverso brevetti, marchi e diritti d'autore al fine di incoraggiare la creazione e la diffusione di soluzioni innovative che possano migliorare tangibilmente la qualità della vita dell'intera comunità. Rappresenta un'opportunità per attrarre risorse economiche utili a trasferire l'innovazione alla sanità creando valore aggiunto per il futuro della ricerca medica
I BREVETTI attualmente in portafoglio sono:
1. “Dispositivo Universale per Anestesia Spinale Continua” – Dispositivo medico ideato per la pratica dell’anestesia spinale continua: ha l’obiettivo di evitare le difficoltà dovute alla fase preparatoria di ricerca dello spazio peridurale, mantenendo, allo stesso tempo, la comodità di una iniezione sub-aracnoidea normale. Il kit creato sintetizza tutti i vantaggi degli altri dispositivi in commercio senza gli svantaggi, per poter accedere direttamente allo spazio sub-aracnoideo.
2. “Formulazioni in gel per la somministrazione orale di farmaci, in pazienti disfagici” – Formulazione in gel contenente principi attivi idrosolubili, prevalentemente disponibili nel mercato farmaceutico come forme di dosaggio solide orali: come, ad esempio, compresse o capsule a rilascio immediato, oppure polveri o granulati ad uso orale. L’invenzione soddisfa quelle particolari esigenze di pazienti con anomalie nel processo di deglutizione. La disfagia è quella particolare condizione patologica relativa alle problematiche dell’atto deglutitorio.
|
|
Technology Transfer Process
|